Abstract
Introduction Novel immunotherapies have changed the treatment paradigm for several oncohematologic diseases. In AML, these strategies have found limited application so far, in part due to incomplete understanding of bone marrow (BM) immune microenvironment. Several studies have identified T-cell exhaustion and senescence as two dominant states of dysfunction. We hypothesized that T cell dysfunctional states will predict response to induction chemotherapy in patients with AML.
Methods We analyzed serial BM samples from healthy donors (HD); and AML patients responding (Res) and not responding (NonRes) to chemotherapy. We performed flow-cytometry in 10 Res, 6 NonRes (at diagnosis and at time of response assessment) and 12 HD. We subsequently performed single-cell RNA sequencing (scRNAseq) to analyze BM of 2 HD, 3 Res, and 2 NonRes at diagnosis and response assessment. This was followed by a scRNAseq-guided 26-color spectral flow-cytometry panel (12 Res and 10 NonRes). In-vitro assays were conducted to study the impact of blasts and metabolites on CD8+ T cells differentiation.
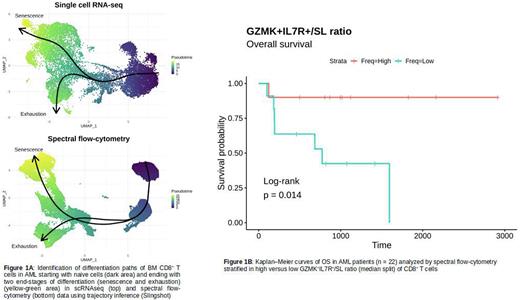
Results Flow-cytometry identified a PD1+CD28+ CD8+ T-cell subset significantly (p<0.01) increased in Res versus NonRes at baseline and post-chemotherapy. Unsupervised clustering of scRNAseq data identified 6 main CD8+ subsets: naive, GZMK+IL7R+, intermediate GZMK+ (int-GZMK), intermediate(Int), senescent-like (SL), and terminally exhausted (Ttex). The GZMK+IL7R+, int-GZMK and Ttex expressed ITGAE and ENTPD1, supporting the idea that these subsets are tumor reactive and exhaustion is antigen-driven. Additionally, GZMK+IL7R+ cells had a progenitor-exhausted signature expressing stemness (TCF7, IL7R), anti-apoptotic (BCL2) and exhaustion (PDCD1, TIGIT) markers. This stem-like signature may allow long-term in-vivo persistence and anti-tumor immune surveillance consistent with the significant increase of GZMK+IL7R+ and Int-GZMK in Res vs NonRes. Conversely, Ttex and SL were significantly increased in NonRes. Trajectory inference algorithms identified two pathways with different end stages (SL; Ttex) demonstrating that senescence and exhaustion coexist but are different states in AML patients (Figure 1A). At trajectory's divergence, GNLY, NKG7, PRF1 and GZMB were the most upregulated genes in the "senescent” lineage while GZMK was upregulated in the "exhaustion” lineage. At the clonal level, the most expanded subsets were GZMK+IL7R+ and int-GZMK in Res and SL in NonRes, revealing a strong relationship between CD8+ T-cell subsets abundance and clonal expansion. The same subsets were confirmed by spectral flow-cytometry. GZMK+IL7R+cells were significantly increased in Res vs NonRes and SL where significantly increased in NonRes vs Res. In this larger cohort we corroborated at the protein level the differentiation path found in scRNAseq, confirming senescent and terminally exhausted CD8+ T cells as end differentiation T-cell states in AML (Figure 1A). Patients with a higher-than-median CD8+ GZMK+IL7R+/SL had a significantly (p<0.05) prolonged overall survival, affirming the clinical relevance of the differential abundance of the identified subsets in AML (Figure 1B). Gene-set enrichment analysis on scRNAseq showed increased hypoxia in NonRes VS Res, suggesting that anaerobic (non-oxidative) metabolism could promote senescence or terminal exhaustion. In vitro experiments with flow cytometry-sorted BM T cells and AML blasts demonstrated that adding lactic acid promotes CD8+ T cells senescence (p<0.05) whereas sodium bicarbonate prevents the development of senescence (p<0.05). These effects appear to be AML blast-specific since co-culture of HD T cells with AML blasts resulted in similar phenotypic changes.
Conclusion Our study provides novel insights in understanding T-cell differentiation in the BM microenvironment of AML patients. We found that terminal exhaustion and senescence are derived from two different differentiation paths and that the ratio between stem-like and senescent CD8+ T cells is predictive of survival. Based on our scRNAseq and in-vitro results, we hypothesize that blasts and diverse metabolic microenvironments in Res and NonRes may have a different impact on T-cell differentiation and be responsible for different patients outcomes. Further studies could explore strategies that promote stem-like functional T cells to gain long-term AML control.
Disclosures
Gojo:Ono Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Clearview: Membership on an entity's Board of Directors or advisory committees; Certara: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Immunogen: Membership on an entity's Board of Directors or advisory committees; Celgene: Other: Research support; Genentech: Other: Research support; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Research support; Amphivena: Other: Research Support; Merck: Other: Research support; Amgen: Membership on an entity's Board of Directors or advisory committees, Other: Research Support; Johnson & Johnson: Membership on an entity's Board of Directors or advisory committees. Luznik:WindMil Therapeutics: Patents & Royalties; Genentech: Other: Clinical Trial support, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Rubius Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Precision Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; Talaris Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal